Kalorama Worldwide IVD Report 14th Edition Published
Kalorama Information, specialists in market research data in the in vitro diagnostics market since 2001, has now published its latest (14th) edition of their comprehensive market report. The market for IVD products including instruments and reagents is $117 billion dollars. Kalorama’s 1,500 + page report is used by top companies in the industry and by prospective entrants, investors and entrepreneurs.
The main finding this year: Not only have test markets for traditional medical tests recovered from the pandemic and related lockdowns, but COVID-19 testing volumes persisted at the same time, swelling the IVD market.
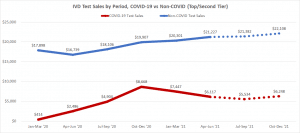
(dollar values and data points and more information included in the report)
In addition to this overall trend, the following are notable:
- IVD is one of the Fastest Growing Healthcare Markets – In 2001, Kalroama measured the in vitro diagnostics market* as $25 billion. The Market is now over $117 Billion.
- Roche is the #1 in vitro diagnostics company, Abbott Diagnostics is #2 and exceeded previous market share levels due to COVID-19 and other infectious disease test sales. Danaher companies (especially Beckman Coulter, Cepheid and Radiometer brands), Siemens Healthineers and Thermo Fisher, bioMerieux, BD, Bio-Rad and other companies are large competitors in this market.
- Recovery in Testing Markets Outside of COVID-19 – as doctor / provider visits spike in the U.S. and abroad, segments hurt by the lack of preventative visits during lockdowns are back: cancer tests and coagulation are seeing double digit growth 2020-2021.
- New Technologies – Molecular test technologies were in their infancy when Kalorama started measuring the market; they played a role in the detection of HIV. Now there are 30 billion dollars worth of molecular test sales.
- COVID-19 on Menus – With eradication not a likely possibility at this point, Kalorama’s analysts include forecasts for COVID-19 as part of the respiratory test “menu” [in multiplex and single tests] until 2026.
*in vitro diagnostics – (latin in glass) any non-microscopy medical testing used for prognosis, diagnosis or drug selection for which a sample is taken from a human patient.
F O R M O R E I N F O R M A T I O N
This is a fraction of the information available in this extensive offering. Reserve your copy of this extensive report. Speak to us today about subscriptions to all Kalorama Information products, other market research and databases that can pinpoint instrument locations in the United States.
The Worldwide Market for In Vitro Diagnostics, 14th Edition
https://kaloramainformation.com/product/the-worldwide-market-for-in-vitro-diagnostic-tests-14th-edition/

