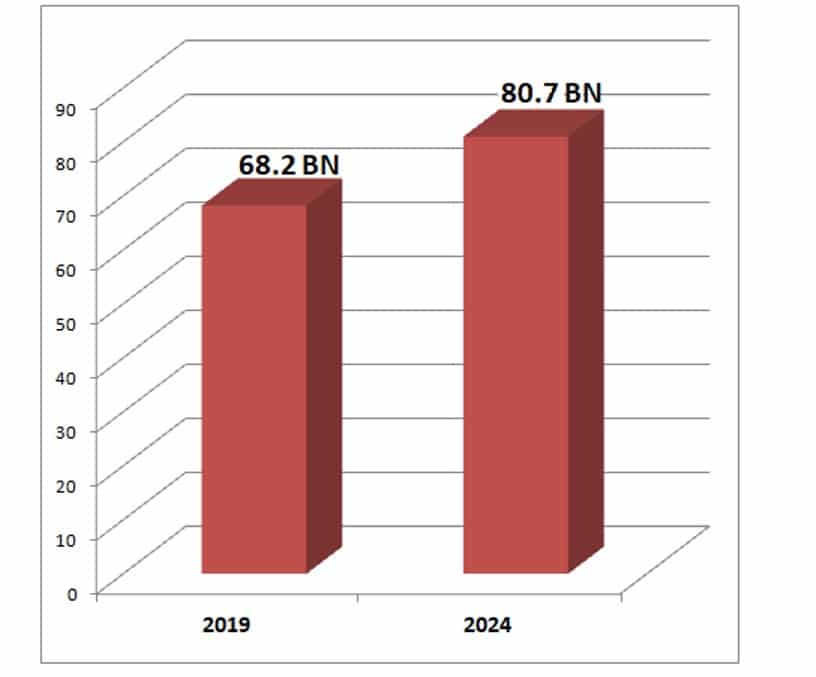
IVD Procedures Reach 68 Billion
The global volume of in vitro diagnostic (IVD) procedures has reached over 68 billion and is forecast to increase 3.4% annually to 81.0 billion in 2024, according to Kalorama’s new report on procedure volumes. These statistics are important for business planning, particularly for new products. The same timeframe will see worldwide sales of IVD products expanding 4.3% per year to over $85.2 billion.
The United States will account for the largest volume of IVD procedures implemented worldwide. The U.S. will maintain a high, diverse level of tests based on its advanced medical delivery system, high healthcare spending intensity, and widespread health insurance coverage of the population. Moreover, its volume of IVD procedures will rise as patient care strategies place an increasing emphasis on early disease detection.
Per capita IVD procedures will remain high in Australia, Canada, Western Europe, Japan, South Korea, and other developed economies based on advanced, widely accessible medical communities and the universal or near universal coverage of residents for medical benefits. However, in most countries, the pursuit of stricter cost containment strategies by government health insurance plans will moderate overall growth in patient testing volume.
The volume of IVD procedures will rise at a fast pace throughout the developing world as countries upgrade and expand medical delivery systems. Reflecting population size and improving availability and accessibility of healthcare services, China and India will perform the largest number of tests. Because of cost constraints and imbalances in healthcare resources, IVD procedures in the developing countries will remain concentrated in basic assays and health screens.
Kalorama’s report provides information on several different segments of the in vitro diagnostics market. Kalorama’s report can be found at: https://kaloramainformation.com/product/ivd-test-procedure-volumes-2019-2024/


