The Kalorama Clinical Masterfile – Definitive Information on U.S. Clinical Diagnostics Instruments
Need Data on U.S. Instrument Placements? With the Clinical Masterfile, You can Know the Instrument Installations, Analyzer Types, Budgets at U.S. Locations.
The Clinical Masterfile is a database of:
>41,000 Laboratory Analyzers
>5,300 Facilities
It is a sold as a12 Month License, with Quarterly Refresh so you are assured of up-to-date information.
Information is continuously gathered by phone and online reporting system with pathologists and lab managers at US CLIA labs.
Find Targets, Verify Your Sales Team’s Data, Locate Competition Weak Points, Benchmark Your Success. Linkable to IDN Data
- Supplements and verifies information collected by manufacturers’ sales team
- Provides opportunities to target labs with old or outdated equipment
- Helps you locate where the competition is strong or vulnerable
- Can be linked to sources of IDN data so that you can understand the resources of an entire IDN; critical to the current healthcare environment.
- Provides geographic information from ESRI data which can be used to target areas with the highest population, lab or imaging expenditures or growth in lab or imaging expenditures
Data collected includes (coverage varies by modality):
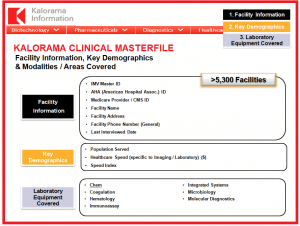
- 1. Facility Information
IMV Master ID, AHA (American Hospital Association) ID, Medicare Provider (CMS) ID, Facility Name, Facility Address, Facility Phone Number (General), Last Interview Date - 2. Key Demographics
Population Served, Healthcare Spend (specific to Laboratory) ($), Spend Index - 3. Modalities / Areas CoveredChemistry, Coagulation, Hematology, Immunoassay, Integrated Systems, Microbiology, Molecular Diagnostics
- 4. Installed Equipment
Manufacturer, Model + Name / Number, Year Installed
Department Location - 5. Contact Fields
Name, Title, Institution ID (note that Client ID number can be added, if applicable)
See a Demo and Discuss the Clinical Masterfile:

