Growth in Molecular Diagnostics, Beyond COVID-19
COVID-19 is not over, but test sales are clearly on the decline, and IVD companies for the most part have adopted a focus on other test areas to beat the negative trend. Abbot, Roche, Hologic and Quest are among the diagnostic testing companies that have reported 2022 results with COVID-19 losses softened by gains in other tests areas. Their financials showed COVID-19 revenues sagged while other revenues grew. For instance Quest grew its base revenues 5% while COVID-19 testing dropped 48%, softening the blow. For Roche revenues were flat, with COVID-19 declines met almost equally by gains in chemistry, and point of care. This trend, of substituting COVID-19 for non COVID-19 revenue in the market largely fits into predictions made by Kalorama in our Worldwide Market for Molecular Diagnostics and our Worldwide Market for In Vitro Diagnostics, 15th Edition. Molecular diagnostics are useful in many disease areas and will continue to see sales.
What are those areas? Our report The Worldwide Market for Molecular Diagnostics details some of these areas.
Infectious Diseases -HIV, STI and Respiratory
HIV testing based on molecular methods is becoming a mature market segment. Yet HIV is still a problem in the world. The number of new HIV infections in the U.S. has been dropping — down from 41,200 in 2012 to 38,300 in 2017 — but fewer than half of adults in the U.S. have ever been tested for the virus. Most of the growth and innovation in the segment currently focus on antiviral therapy, which includes testing for viral load, viral tropism, and drug resistance. While the need for effective HIV screening and diagnosis is high in many developing markets, accessibility to such effective methods remains a challenge in most of these countries. Clinical approaches to HIV in the United States, Europe and other developed countries emphasize molecular testing as a tool for the management of chronic infection and achieving viral suppression. Recommendations released from the U.S. Preventive Services Task Force (USPSTF) continue to strongly advise HIV screening for adolescents and adults, and for the first time they support preventive treatment for those at high risk. The guidance could boost volume for blood tests.
The USPSTF, a volunteer group of independent experts that makes evidence-based assessments for disease prevention, published two recommendation statements on HIV screening and preventive treatment in the Journal of the American Medical Association (Vol. 321:22, pp. 2203-2213). The USPSTF’s last guidance on this topic was published in 2013. The group gave an “A” recommendation to screening for HIV in adolescents and adults between the ages of 15 and 65 years and for all pregnant women. Furthermore, it advised screening for people younger than 15 and older than 65 who are at risk of getting infected. The USPSTF did not find enough evidence to set screening intervals, but it concluded that “repeat screening is reasonable for persons known to be at increased risk of HIV infection,” including sexually active men who have sex with men, people who inject drugs, and/or those who are involved with commercial sex work.
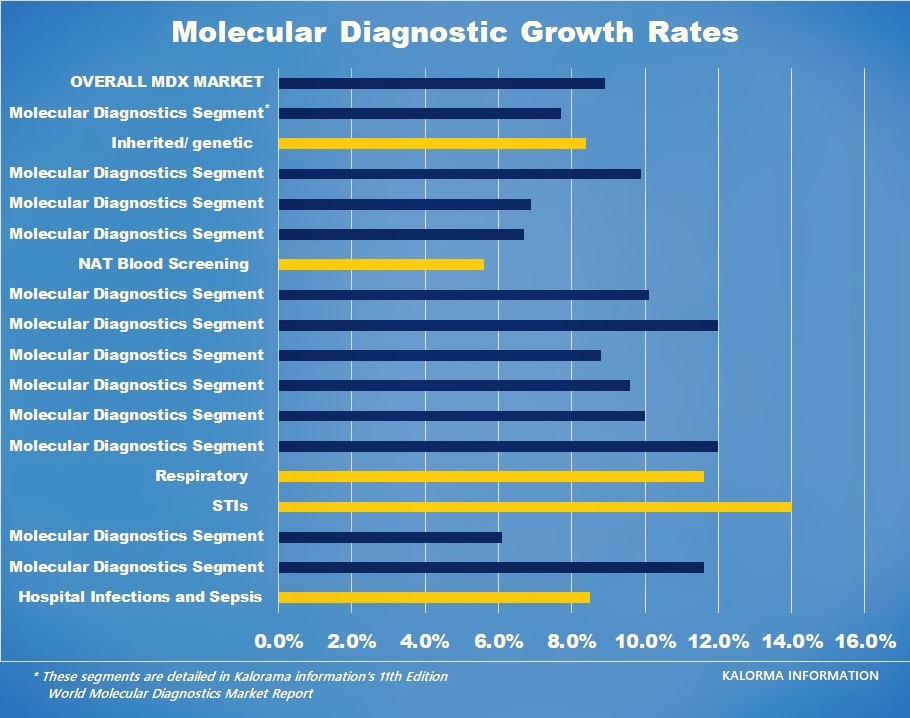
In addition Kalorama’s report marks both STIs and non-COVID-19 respiratory as key growth areas.
The chart above shows growth in only a limited amount of segments. Kalorama reports, including The World Market for Molecular Diagnostics, 11th edition provides additional growth segments and market sizes in $USD
Inherited Diseases – Biomarker Improvement and Growing Acceptance of Testing
Alzheimer’s disease (AD) is an aging-associated disease that manifests as dementia. The progression of the disease is associated with declines in memory and cognition, impaired judgment, and deterioration of personality. The disease afflicts over 5 million in the United States and is expected to reach 16 million by 2050.
Certain alleles of the apolipoprotein E (ApoE) gene are known as major risk markers for the development of AD. The advent of economical whole genome and exome sequencing in research has spurred investigation into weaker or secondary genetic loci for the disease. Whole genome sequencing of AD patients has identified several gene markers including CLU, CRI, PICALM, BIN1, and CNTN5.
The American Academy of Pediatrics and the American College of Medical Genetics recommend that all children diagnosed with autism be tested for Fragile X Syndrome and other chromosome abnormalities. These tests can help find genetic explanations in more than 10% of autism cases. Many more autism studies such as Kaiser Permanente’s Autism Research Program are comprehensively testing patients diagnosed with autism as well as their families.
Cardiovascular disease, including heart attack and stroke, is the leading cause of death in the world and claims more lives than all forms of cancer combined. The number of deaths from cardiovascular disease (CVD) worldwide is projected to reach approximately 24 million by 2030. Approximately 80% of CVD-related deaths are in low- and middle- income countries. In developed countries such as the United States, CVD is one of the leading killers. Globally, CVD is on the rise as a result of sedentary lifestyle; changes in diet related to development (higher consumption of animal products); and smoking.
NAT Blood Screening – Continue to Replace Immunoassays
In contrast, less growth will be seen in our NAT Blood Screening category. Molecular blood screening tests, also known as Nucleic Acid Test blood screening tests, are covered in this section. Though a fair amount of global blood screening is conducted by immunoassays, in major markets this category is growing.
Immunoassays are largely used to screen blood donations in developing nations as a routine approach. However, as a result of concerted efforts to make blood product transfusions safer, there is an increase in nucleic acid test (NAT) screening to test for common pathogens. Developing countries are replacing immunoassays with molecular tests, especially for HIV, hepatitis, etc. Although infected donors can still test negative with NAT, the length of time between initial infection and a positive test result is shorter, resulting in fewer false negatives.
According to the WHO, 13,000 blood centers in 176 countries collect whole blood, with about 79% of these units collected in high-income countries that have the resources to operate fully organized blood transfusion services and that screen donated blood for transfusion transmissible infections (TTIs), HIV, hepatitis B, hepatitis C, and syphilis. The WHO reports that almost all reporting countries screen donated blood for TTIs but there are 13 countries that are not able to screen all donated blood for one or more of the above infections. Irregular supply of tests kits is the most common barrier to performing these tests.
The pioneers in the molecular diagnostics field include Roche Diagnostics, Gen-Probe, and Becton Dickinson. The main focus of the initial molecular diagnostics products was infectious disease including HIV, Chlamydia Trachomatis/Neisseria Gonorrhoea (CT/NG), and TB. These diseases are still critical components of the market. Recently growth in molecular diagnostics methods has also been coming from inherited diseases, cancer, coagulation, and other areas.

