Healthcare Advances, Investments Support Laboratory Information System (LIS) Growth
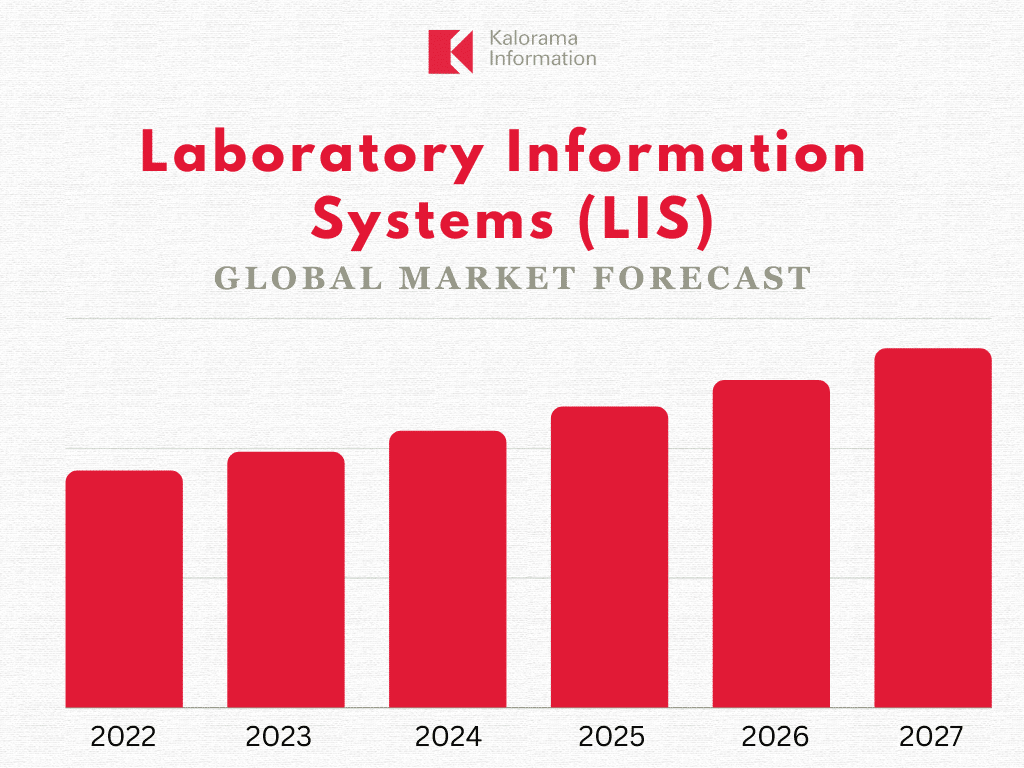
The global market for LIS is forecast to increase at a high single-digit compound annual growth rate (CAGR) between 2022 and 2027, reveals leading medical market research publisher Kalorama Information in the new report World Market for Laboratory Information Systems (LIS): Software, Hardware, and Implementation for Clinical Labs, 2022-2027.
Advances in healthcare will function as the driving force behind the growth of the global LIS and laboratory information management systems (LIMS) markets. A growing need for integrated information systems and new investments by medical and healthcare IT industry participants are aiding this growth. However, the shortage of skilled professionals, the high cost of services and equipment maintenance, and interoperability problems may restrict some market growth.
The market for clinical lab LIS will grow at a strong pace, spurred on by the desire of clinical diagnostic laboratories to become more efficient and profitable. Many clinical labs have installed some form of LIS and automation and intend to make future purchases to further optimize their operations.
The market for LIMS in drug discovery efforts also has grown in recent years. Software and hardware have become important in improving laboratory operations. Management software and automation find use in many laboratory processes, ranging from the capping and decapping of sample bottles to high throughput screening. The greater use of LIMS, LIS, and laboratory automation to manage next-generation sequencing (NGS) data and molecular testing has helped spur sales as well.
Although the drug industry has curtailed its spending in general, the demand for LIMS is still growing due to new products and automated solutions for newer techniques, such as microarrays, as well as cell-based assays. However, laboratory informatics and automation have not necessarily led to the increased rate of commercialization of new pharmaceutical compounds. Yet there will be growth for enterprise LIMS as companies synchronize their laboratory operations at different sites.
The typical LIS or LIMS implementation has a life cycle of at least five to 10 years due to the costs of implementation and complexities involved—training, support and the like. Users today have high expectations concerning functionality. There are a large number of vendors who now offer both tailored systems as well as commercial-off-the-shelf (COTS) with specific functionality. Many vendors provide regularly scheduled product enhancements that meet changing market demands.
Many large laboratory networks desire large, stable system vendors with a proven track record of success in delivering large-scale implementations. Some in vitro diagnostic (IVD) vendors offer their own LIS products that they bundle for sale with their instrumentation. Such LIS can be economically attractive. In this manner, some LIS IVD vendors more competitively price their systems when they bundle them with reagent sales.
About the Report
World Market for Laboratory Information Systems (LIS): Software, Hardware, and Implementation for Clinical Labs, 2022-2027
This new report from Kalorama Information examines the current and potential world opportunity for healthcare laboratory information system (LIS) software, hardware, and implementation. The focus of this report is on the market for LIS in clinical diagnostic and healthcare-related labs, although LIMS in pharmaceutical drug discovery laboratories, and other drug research and development labs, are discussed as well.
For more information or to purchase World Market for Laboratory Information Systems (LIS): Software, Hardware, and Implementation for Clinical Labs, 2022-2027, visit: https://kaloramainformation.com/product/world-market-for-laboratory-information-systems-lis-software-hardware-and-implementation-for-clinical-labs-2022-2027/.
About Kalorama Information
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Kalorama Information produces dozens of reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Kalorama Information’s studies feature independent primary research conducted by experienced analysts. Researchers build their market analysis independently from published databases, validating data with inside industry contacts and extensive secondary research, so you can have confidence that you’re getting your information from the most trusted source in the industry!

