Personalized Medicine Key to IVD Procedure Volumes’ 10% Annual Growth
The in vitro diagnostic (IVD) industry is on track again after the disruptions of the pandemic. The overall volume of IVD procedures will rise steadily throughout most of the world, boosted by aging population trends, proliferating epidemiological patterns, increasing availability of healthcare funding, and widespread adoption of patient care strategies that emphasize early disease detection and preventive medicine. As a result of these key factors, the global volume of IVD procedures is forecast to increase almost 10% annually from 2023-2028, according to the diagnostic market research report In Vitro Diagnostic (IVD) Procedure Volumes (Chemistry, Immunoassays, Hematology, Microbiology, Histology, Point of Care), 2023-2028.
The September 2023 report, currently for sale by leading medical market research publisher Kalorama Information, presents projections covering the 2023 to 2028 period for:
- the global number of IVD procedures by type (self and professional point-of-care, clinical chemistry, immunoassay, molecular, hematology, coagulation, microbiology, blood banking, and histology/cytology);
- the total number of IVD tests by region and selected countries;
- and the average and total worldwide cost of various IVD tests.
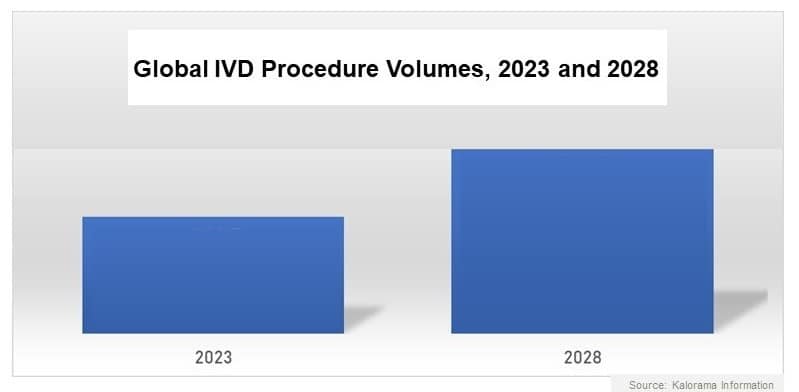
Kalorama Information forecasts that other factors contributing to growth will include ongoing improvements in healthcare delivery systems, an increasing volume of inpatient and outpatient activity, and heightened attention on reducing the incidence of healthcare-associated infections (HAI) in hospitals and other healthcare facilities.
Beyond this, one of the most intriguing trends to watch is personalized medicine, which is also known as precision medicine. Personalized medicine has a fairly broad meaning, but it is medicine that uses an individual’s unique genetic profile to guide decisions about prevention, diagnosis and treatment of disease.
Personalized medicine is an innovative approach to tailoring treatment to the individual characteristics of each patient. This is a relatively new field of medicine that combines pharmacology and genetics, and it is picking up steam.
IVD is an integral part of personalized medicine as it provides critical information needed to make treatment decisions. Specific examples where IVD devices provide personalized medicine include blood glucose tests, pregnancy tests, cancer screening and in-home HIV testing.
COVID-19 was very instrumental in creating a sharp rise in the demand for IVD products causing an unprecedented surge in polymerase chain reaction (PCR), next-generation sequencing (NGS), and serology-based rapid tests. The pandemic also demonstrated the value of these products and helped to create a very supportive regulatory environment.
One reason that personalized medicine is advancing is that patients are demanding more personalized care—the one-size-fits-all model is not satisfying the expectations of the patients. Patients are becoming increasingly more engaged in personally-directed care through the use of biometric indices such as smartphones or smart wearables, i.e. watches, rings or other monitors. These devices are generating a massive amount of data providing insights into patient health and providing physicians with patient-specific data. A recent testament to the growing popularity of wearables in healthcare was an appearance by Abbott Laboratories CEO Robert Ford at the HLTH 2023 conference in Las Vegas earlier in October discussing the company’s expansion into a new market: consumer wearables.
The growing evidence of technologies that provide personalized data is likely to lead to better patient outcomes. Many of the IVD tests are benefiting from the uptake of personalized medicine which is expected to continue to increase.
For more information or to purchase In Vitro Diagnostic (IVD) Procedure Volumes, 2023-2028, visit Kalorama Information’s website at: https://kaloramainformation.com/product/ivd-procedure-volumes-2023-2028/.
About the Report
In Vitro Diagnostic (IVD) Procedure Volumes, 2023-2028 is an essential resource for the IVD business planner. It is intended to serve as a companion piece to Kalorama’s flagship IVD publication—The Worldwide Market for In Vitro Diagnostic Tests, 16th Edition.
The market analysis in this report offers calculations and estimates of both the existing number of procedures for scores of diagnostic tests and the future potential. In the process, pricing analysis is also performed.
About Kalorama Information
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Kalorama Information produces dozens of reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Kalorama Information’s studies feature independent primary research conducted by experienced analysts. Researchers build their market analysis independently from published databases, validating data with inside industry contacts and extensive secondary research, so you can have confidence that you’re getting your information from the most trusted source in the industry!



