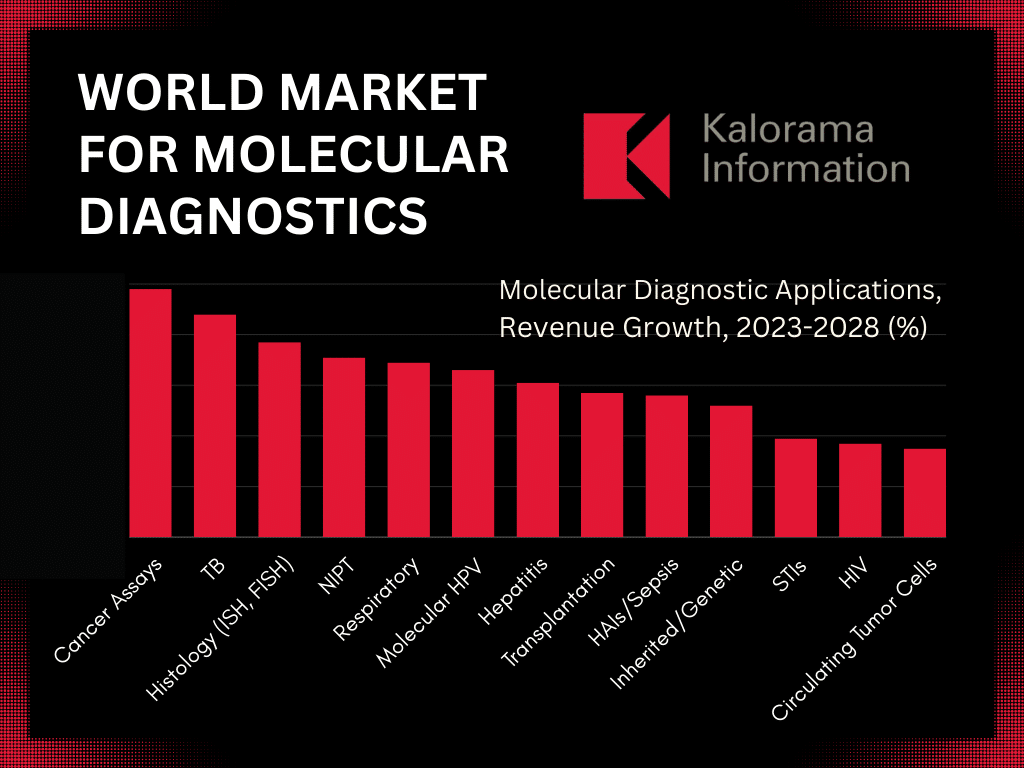
Molecular HPV Testing: 6.6% Revenue Growth 2023-2028
January is Cervical Health Awareness Month, a reminder that more than 95% of cervical cancer cases are attributed to the human papillomavirus (HPV), according to the World Health Organization (WHO).
HPV testing is a key segment of the double-digit, multi-billion-dollar global molecular diagnostic testing market, according to the new report The World Market for Molecular Diagnostics, 12th Edition published by leading medical market research firm Kalorama Information. The report reveals that through 2028, molecular HPV testing will grow 6.6%—outpacing growth in several other notable molecular testing segments including HIV, STIs, and genetic/inherited diseases.
To combat and prevent cervical cancer effectively, two primary approaches have proven to be highly successful:
- Prophylactic Vaccination against HPV:
- HPV vaccines are designed to protect against the most common high-risk HPV types that cause cervical cancer.
- Vaccination is a preventive measure and is often administered before potential exposure to the virus, ideally before the onset of sexual activity.
- HPV vaccination has demonstrated significant success in reducing the incidence of cervical cancer and other HPV-related diseases.
- Screening and Treatment of Pre-cancer Lesions:
- Regular screening tests, such as Pap tests and HPV tests, are instrumental in detecting pre-cancerous changes in cervical cells.
- Early detection allows for timely intervention and treatment of pre-cancerous lesions, preventing their progression to cervical cancer.
- Screening, when followed by appropriate treatment, is a highly effective strategy in reducing cervical cancer risk.
Both prophylactic vaccination against HPV and routine screening with subsequent treatment of pre-cancerous lesions contribute significantly to cervical cancer prevention efforts. These measures are not only effective in reducing the incidence of cervical cancer but are also considered cost-effective public health interventions. Regular vaccination, coupled with screening programs, plays a crucial role in achieving the goal of preventing the majority of cervical cancer cases. Recent news regarding HPV screening involved global healthcare leader Abbott, who was granted approval by the U.S. Food and Drug Administration (FDA) for its molecular HPV screening solution in November 2023.
The majority of HPV testing is performed in reference labs and clinical molecular labs using high-throughput systems as part of a liquid cytology workflow. Generally, very little HPV testing is performed near-patient with only some atypical OB/GYN clinics and other outpatient care providers performing the tests in-house. Because its detection is used towards a cancer-preventing purpose, HPV testing is an important part of oncology diagnostics as well as infectious disease in the molecular diagnostics market.
The first category where molecular diagnostics earned their place was infectious disease. In this category, specific results were needed for treatment decisions. The pioneers in the molecular diagnostics field include Roche Diagnostics, Gen-Probe, and Becton Dickinson. The main focus of the initial molecular diagnostics products was infectious diseases including HIV, Chlamydia Trachomatis/Neisseria Gonorrhoeae (CT/NG), and tuberculosis (TB). These diseases are still critical components of the market. Recent growth in molecular diagnostics methods has also been coming from cancer, TB, ISH/FISH, and other areas.
Over the last 25 years, molecular tests have been used in just about every segment of laboratory medicine. Covering diseases for which treatment decisions require accurate results, infectious disease testing makes up the bulk of molecular test sales. The COVID-19 crisis brought increased media attention and general awareness to the molecular testing technologies being utilized for infectious diseases. Today, molecular diagnostics has evolved into an essential instrument in clinical medicine, influencing various facets of healthcare, according to Kalorama Information.
For more information, purchase The World Market for Molecular Diagnostics, 12th Edition by clicking HERE.
About Kalorama Information
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Kalorama Information produces dozens of reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Kalorama Information’s studies feature independent primary research conducted by experienced analysts. Researchers build their market analysis independently from published databases, validating data with inside industry contacts and extensive secondary research, so you can have confidence that you’re getting your information from the most trusted source in the industry!

