IVD Trends 2023-2028: Emerging Growth Markets in Asia, Latin America, and the Middle East
In vitro diagnostic (IVD) testing is truly global as it has impacted every country in the world due to COVID-19 and it will continue to play an ever-increasing role in the practice of medicine. Social and economic events in selected countries will continue to influence the IVD market, but overall the IVD market plays an important role in the evolution of the healthcare industry worldwide, especially in emerging regional markets. This is according to the market research report The Worldwide Market for In Vitro Diagnostic Tests, 16th Edition by leading firm Kalorama Information.
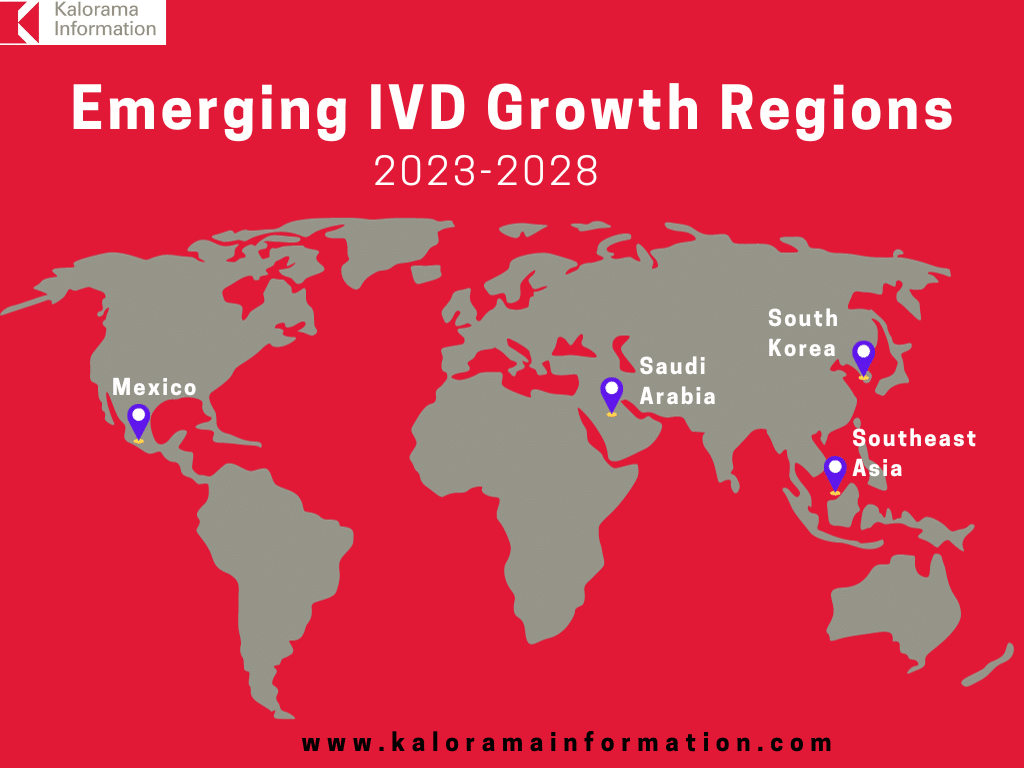
There aren’t too many countries or regions in the world left where the IVD market growth will be double the world IVD market growth. South Korea is a rare example. With a population of 51.7 million, a per capita GDP higher than China and closing in on Japan, this is a large and growing IVD market and will be one for the near future. A national health insurance program covers virtually all residents for basic patient care services, including diagnostic testing. Similar to Japan, the country experiences a relatively high incidence of age-related medical conditions. Kalorama Information estimates the IVD market in South Korea to be $464 million and with 5.9% growth will reach nearly $617 million by 2028. There is a catch, South Korea is an IVD-focused country with in-country global competitors such as Seegene and SD Biosensor.
A growing number of nations in Southeast Asia—including Malaysia, Thailand, Cambodia, and Indonesia—now offer basic medical coverage to their citizens. This trend is leading to fast expanding demand for medical devices, drugs, and diagnostic test kits and reagents, benefiting Western companies marketing their products in the region. These countries will boast higher growth than the world market.
Mexico’s IVD market is worth about $743 million in 2023, and with 3.6% compound annual growth rate (CAGR) the market will reach $886 million in 2028. Growth is supported by continued acceptance of advanced equipment and growing distribution channels. Segments with strong demand include immunoassays, microbiology and histology/cytology.
Saudi Arabia is another emerging IVD player. Trends in the sales of these products will reflect procedures provided by 557 hospitals, 98,400 physicians, and 9,800 clinical laboratories. Driving the IVD market in Saudi Arabia included 4.9 million hospital admissions, 18.3 million inpatient days, 5.4 million surgical procedures, and 188 million outpatient episodes. Health and living standards in Saudi Arabia are very good. About 85% of the population has ready access to medical providers. The market for diagnostics in Saudi Arabia is estimated at $591 million in 2023 and is expected to grow 5.9% annually to $789 million by 2028.
For more information, purchase The Worldwide Market for In Vitro Diagnostics here.
About “The Worldwide Market for In Vitro Diagnostics”
This report, The Worldwide Market for In Vitro Diagnostics, now in its 16th Edition, details IVD activity on a global scope. The report looks at the entirety of the in vitro diagnostic market and makes forecasts, accesses trends, and scrutinizes vendor activities.
The report discusses tests and technologies that are currently available and those that are expected to take their place. Generally, current products and technologies establish the standard of care and its value to payers. Meanwhile, market analysis in this report covers world markets for in vitro diagnostics; however, the reader will find a bias toward the developed areas of the globe—North America, Japan and Western Europe.
The report also covers more than twenty core market segments, including:
- Blood Bank Molecular
- Blood Bank Screening
- Blood Grouping/Typing
- Circulating Tumor Cells
- Clinical Chemistry
- Coagulation
- Continuous Glucose
- COVID-19
- D-dimer
- Drugs of Abuse
- HbA1c Immunoassays, Lab-Based
- Hematology
- Histology/Cytology
- HPV, molecular
- Immunoassays, Infectious Disease
- Immunoassays, Other
- Mass Spectrometry
- Microbiology (ID/AST)
- Molecular Microbiology
- Nucleic Acid Assays
- POC Professional
- POC, OTC diabetes
- POC, OTC Other
Why Kalorama Information?
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Kalorama Information produces dozens of reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Kalorama Information’s studies feature independent primary research conducted by experienced analysts. Researchers build their market analysis independently from published databases, validating data with inside industry contacts and extensive secondary research, so you can have confidence that you’re getting your information from the most trusted source in the industry!



