Liquid Biopsy on Frontlines as Prostate Cancer Concerns Escalate
Each year, tens of thousands of men succumb to prostate cancer, a disease with an almost 100% survival rate when diagnosed early. According to a recent article by Fortune, rates of prostate cancer, America’s second-deadliest cancer in men, are on the rise.
Since 2014, the U.S. has seen a 3% annual increase in diagnoses of prostate cancer and a 5% annual increase in advanced-stage prostate cancer diagnoses. The biggest risk factor for prostate cancer appears to be age, and increased occurrence is associated with an aging global population, and rapid uptake of PSA screening.
Prostate health has garnered attention recently, sparked by reports that King Charles III is undergoing treatment for an unspecified form of cancer discovered during his treatment for a benign prostate condition.
Given the various challenges in the screening and management of prostate cancer patients, this area has seen a significant interest for the development of liquid biopsy tests. Such tests are highly needed to replace the low-specificity PSA test that is typically used to refer patients to biopsies, and leads to high numbers of unnecessary invasive procedures and overtreatment.
In the liquid biopsy market, several prostate cancer tests are currently available, with a few more in development, according to the market research report The Worldwide Market for Liquid Biopsy, 6th Edition by Kalorama Information. Most prostate cancer tests are urine-based, as analytes shed by the prostate can be found in high concentrations. Notably, Hologic offers the Progensa PCA3 test, which assesses the likelihood of a positive tissue biopsy by quantifying the overexpression of PCA3 in RNA.
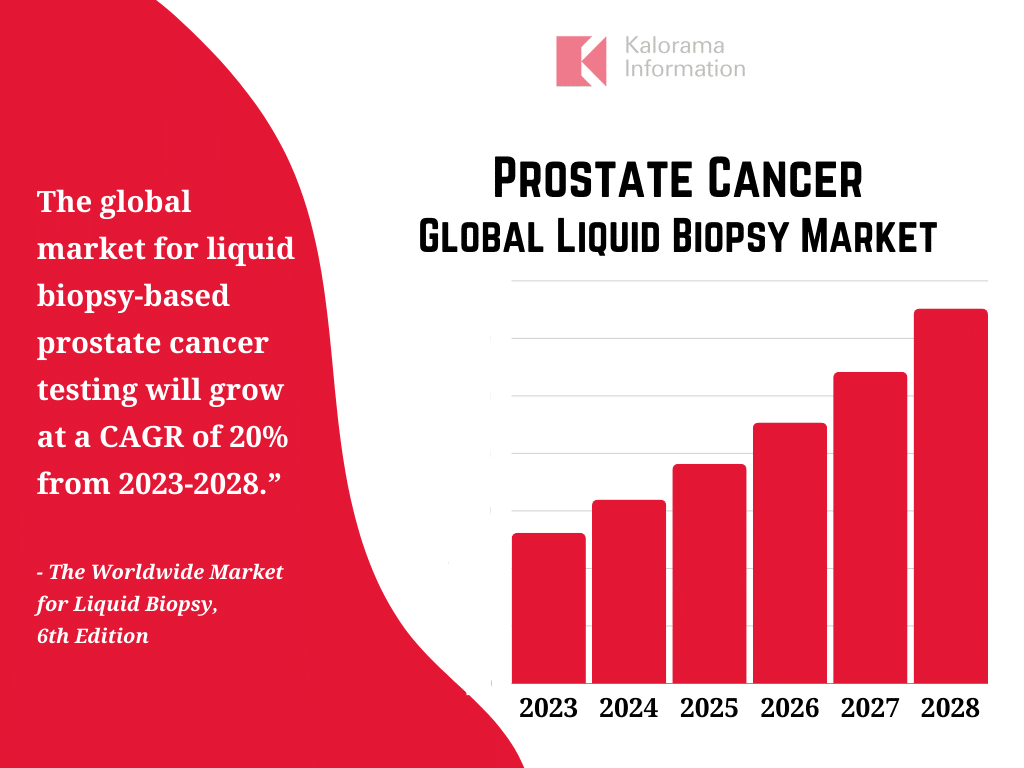
In The Worldwide Market for Liquid Biopsy, 6th Edition, Kalorama Information estimates that the global market for liquid biopsy-based prostate cancer testing surpassed $130 million in 2023. Demand is expected to increase at a compound annual growth rate (CAGR) of 20% from 2023 through 2028.
Liquid biopsies are a set of minimally invasive diagnostic methods that analyze tumor-derived materials that can be found circulating in biological fluids, to provide information for the diagnosis, treatment, and monitoring of cancer. While tissue biopsies and imaging techniques remain the current standards of care in the diagnosis of solid tumors, they have risks and limitations and limitations, some of which can be overcome by the use of liquid biopsy in clinical oncology, as an alternative or complementary technique to the current standards of care.
Liquid biopsy testing has numerous potential applications in clinical oncology, including:
- Early detection and diagnosis/screening.
- Alternative testing when tissue biopsy is challenging or impossible, or when the primary site of metastatic disease is unknown.
- Personalizing therapy and monitoring progress. Molecular characterization of a patient’s disease allows clinicians to select the best course of therapy, monitor its effectiveness, and quickly adjust treatment if resistance develops.
- Monitoring disease progression, tumor evolution, residual disease, and early detection of recurrence.
- Determining the prognosis of the disease.
For more information, purchase The Worldwide Market for Liquid Biopsy, 6th Edition. Click HERE to see the report abstract, table of contents, or to make a purchase.
About the Report
The Worldwide Market for Liquid Biopsy, 6th Edition from Kalorama Information provides an in-depth assessment of the market opportunities from 2023 to 2028. This brand new market research report covers various aspects, including market segmentation by region, different types of liquid biopsy (CTC-based, ctDNA-based, EV and Exosome-based, Multi-Analyte-based, and Other Analytes), and cancer types.
About Kalorama Information
Kalorama Information, part of Science and Medicine Group, is the leading publisher of market research in healthcare areas, including in vitro diagnostics (IVD), biotechnology, medical devices, and pharmaceuticals. Kalorama Information produces dozens of reports a year. The firm offers a Knowledge Center, which provides access to all published reports.
Kalorama Information’s studies feature independent primary research conducted by experienced analysts. Researchers build their market analysis independently from published databases, validating data with inside industry contacts and extensive secondary research, so you can have confidence that you’re getting your information from the most trusted source in the industry!

