Major Molecular Diagnostic Vendors Announce Workshops for AMP
As molecular pathologists, pharmaceutical companies and in vitro diagnostic vendors gather in Baltimore MD for the upcoming Association for Molecular Pathology (AMP) in Baltimore, MD. corporate workshops have been announced. The workshops indicate the direction the industry is going, with new molecular technologies such as mass spec and NGS replacing traditional ones in some cases. Roche, Abbott Diagnostics, Qiagen, Thermo Fisher, Quidel, and Beckman Coulter are among IVD vendors that will exhibit at the meeting and run workshops and presentations.
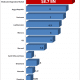
The molecular diagnostics market is estimated at 8.7 billion, per our most recent report on molecular diagnostics:
Molecular pathology vendor workshops that have been announced include:
Next-Generation Sequencing: News of the recent proposed expansion of Medicare coverage for next-generation sequencing testing should be good news for vendors. (https://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=296). Many vendors will present on the topic – Qiagen will focus on the key bottle neck of clinical NGS test interpretation – with a seminar about reducing interpretation times by 85%. Twist Bioscience will present: “Leading the Way in Target Enrichment. Beckman Coulter Life Science will host “Implementation of Automated Comprehensive NGS Panel at a Community Cancer Center” presented by Dr. Qi Wei, Assistant Professor Department of Biomedical Informatics at Vanderbilt University.
Other workshops related to NGS include Asuragen’s which will be highlighting its NGS-in-a-Box solution for DNA and RNA variants. Arc Bio will focus on NGS-based metagenomics for micro labs; and Bio-Rad will be demonstrating orthogonal methods for validation and confirmation of NGS. Archer Dx will focus on blood cancers. The company’s panels can characterize fusions, CNV and other variants. Karius will discuss its test which the company says can identify microbial-free DNA from plasma in patients with severe infection.
Mass Spectrometry: Agena Bioscience will feature “Low Cost, High Sensitivity: The Advantages of MALDI-TOF for Liquid Biopsy and Solid Tumor Profiling.” The company’s MassARRAY system detects genetic variation directly by label-free mass spectrometry. Its highly sensitive, multiplexed UltraSEEK technology, on the MassARRAY system can detect low levels of variants, which can be useful for analysis of somatic tumors.
SMN1/2: Survival Motor Nueron protein is one of a group of proteins called the SMN complex, which is important for the maintenance of specialized nerve cells called motor neurons. Many mutations in the SMN1 gene have been found to cause spinal muscular atrophy. Asuragen will demonstrate AmplideX PCR/CE SMN1/2 Plus Kit, which the company says offers rapid turnaround time, and automated results reporting software, the assay provides a simple and scalable SMN1 solution for all laboratories.
BCR-ABL monitoring: Each year 1.0-1.5 newly diagnosed Chronic Myelogenous Leukemia (CML) patients are identified per 100,000 individuals. CML prevalence is estimated to increase at an annual rate of 4%, and the number of individuals living with this disease will double by 2030. CML is treated with a TKI called imatinib that targets the BCR-ABL fusion protein but assessing treatment efficacy for CML requires a molecular diagnostic. Asuragen will demonstrate their FDA-cleared assay for highly sensitive BCR-ABL monitoring and Cepheid will show its Xpert BCR-ABL Ultra test which automates the entire test process in one cartridge.
Liquid Biopsy: Roche will be demonstrating his Pan Liquid Biopsy Testing AVENIO ctDNA Clinical Research Story: Surveillance & Monitoring All four mutation classes (SNVs, indels, fusions and CNVs) in a single DNA workflow with exceptional sensitivity and specificity. Exactly-matched tissue and ctDNA panels (same genes, gene regions and hybrid-capture workflow) for true concordance. The company says the test reports in 5 days. Illumina’s new oncology menu for for NovaSeq 6000 System, which the company says offers high-throughput testing for liquid biopsy will be presented in another workshop, one of several hosted by the sequencing giant.
POC: Point of care has transitioned to molecular in the last five years; corporate workshops on this topic include Quidel’s on POC testing for influenza, and Roche’s look at centralization and decentralization trends in diagnostic testing.
Carbapenem Resistance: (CRE) or carbapenemase-producing Enterobacteriaceae (CPE) are Gram-negative bacteria that are resistant to the carbapenem class of antibiotics, considered the drugs of last resort for such infections. Meredian Bioscience will hosting a seminar on “Carbapenem Resistance Solutions for a Clear and Present Public Health Threat” The company’s Revogene Carba C assay provides healthcare systems with a diagnostic solution to enable early detection and management of CRE.
LabPulse.com and Kalorama Information will be covering the meeting and providing highlights of important vendor and scientific announcements.